Để sử dụng toàn bộ tiện ích nâng cao của Hệ Thống Pháp Luật vui lòng lựa chọn và đăng ký gói cước.
| THE MINISTRY OF HEALTH | SOCIALIST REPUBLIC OF VIETNAM |
| No. 43/2011/TT-BYT | Hanoi, December 05th 2011 |
ON THE MANAGEMENT OF INFECTIOUS SPECIMENS
Pursuant to the Government's Decree No. 188/2007/ND-CP dated December 27, 2007, defining the functions, tasks, powers and organizational structure of the Ministry of Health and the Government's Decree No. 22/2010/ND-CP dated March 09th 2010, amending and supplementing Article 3 of the Government's Decree No. 118/2008/ND-CP dated November 27th 2008, defining the functions, tasks, powers and organizational structure of the Ministry of Health;
Pursuant to Clause 3 Article 25 of the Law on the Prevention and suppression of infectious diseases;
The Ministry of Health specifies the management of patient specimens related to infectious substances and conditions of the organizations in charge of the management of patient specimens of infectious diseases that contain infectious substances category A:
Article 1. Scope of regulation
This Circular specifies:
1. The management of patient specimens of infectious diseases serving the research, diagnosis, disease treatment and prevention, including:
a) Collection;
b) Storage;
c) Packaging;
d) Transportation;
dd) Retention;
e) Use;
g) Exchange;
h) Destruction.
2. The conditions of the organizations licensed to store, retain, use, research, exchange, and destroy patient specimens containing infectious substances in category A.
Article 2. Interpretation of terms
In this Circular, the terms below are construed as follows:
1. Patient specimens are specimens of blood, serum, plasma, urine, feces, excreta, and other specimens from the people that contain infectious substances and microorganisms pathogenic to humans (hereinafter referred to as specimens).
2. Infectious substances are substances that are known or expected to contain pathogens affecting humans. Infectious substances are divided into category A and category B.
3. Specimens containing category A infectious substances (according to the list in Annex 1 enclosed with this Circular) are specimens that are capable of causing permanent disability, life-threatening, or fatal diseases in humans when expose to it.
4. Specimens containing category B infectious substances are specimens that contain the infectious substances that are not in the list of category A infectious substances in Clause 3 of this Article.
Article 3. Organizations licensed to management specimens
1. Organizations and individuals licensed to test, examine, store, use, and destroy specimens may collect, preserve, store, export, import, test, store, and examine specimens.
2. Organizations and individuals that import, export, and transport specimens must be licensed to do so.
3. Organizations and individuals licensed to test, examine, store, use, and destroy specimens are responsible before law of the quality and safety of the products and their activities.
Article 4. Collection of specimens
1. Persons in charge of collecting specimens must be skilled in collecting specimens.
2. The collection of specimens must ensure the safety and avoid infecting the specimen takers, their co-workers, the laboratory personnel, and patients.
3. The symptoms that need sampling and the specimens taken are specified in Annex 2 enclosed with this Circular.
4. The techniques for collecting specimens are specified in Annex 3 enclosed with this Circular.
Article 5. Storage of specimens
Specimens collected must be stored in an environment and at a temperature specified in Annex 4 enclosed with this Circular before being sent to the laboratory.
Article 6. Packaging of specimens
1. Specimens containing infectious substances in categories A and B must be separately packaged, not together with other goods. The package consists of 03 layers:
a) The primary receptacle (tubes, bottles, vials that contain specimens) must be watertight, leak-proof, and able to withstand a temperature of -40oC to 55oC;
a) The secondary receptacle (bag, box, pack) must be durable, watertight, leak-proof, and able to withstand a temperature of -40oC to 55oC;
The primary receptacle or secondary receptacle must be able to withstand a pressure of 95kPa;
c) The outer receptacle (box) must be hard, . There is a buffer against shock between the secondary receptacle the outer receptacle. The minimum outer dimension of each side is 10 cm.
2. The package of a liquid specimen must have an absorbent lining (sufficient to absorb all the specimen if a crash occurs) between the primary receptacle and the secondary receptacle.
3. When multiple specimens (contained in the primary receptacles) are contained in the secondary, the specimens must be separated in order to prevent contact among them.
4. For the substances that need preserving in cold storage, dry ice or other refrigerant shall be placed around the secondary receptacle.
a) If ice is used, the outer receptacle must be leak-proof.
a) If dry ice is used, the outer receptacle must permit the release of carbon dioxide gas.
c) If liquid nitrogen is used, the primary receptacle and secondary receptacle must be capable of with standing low temperature.
5. The primary and secondary receptacles must be able to with stand the temperature at which specimens are stored.
6. The lyophilized specimens may be contained in primary receptacles made of flame-sealed glass or rubber-stoppered glass vials.
1. The label on each tube of specimen must indicate:
a) Patient’s name and age;
b) Number;
c) Type of specimen (e.g. blood, serum, cerebrospinal fluid, or other type of specimen), collection date;
2. The label on the package must indicate:
a) Consignor’s name and address;
b) Phone number of the responsible person, knowledgeable about the shipment;
c) Consignee’s name and address;
d) The United Nation number (UN2814 for category A infectious substances or UN3373 for category B infectious substances);
dd) Temperature storage requirement;
e) When dry ice or liquid nitrogen is used, the refrigerant, its UN number and the net quality must be indicated;
g) Each type of products must have appropriate labels as prescribed in Annex 5 enclosed with this Circular.
3. For specimens containing category A infectious substances, between the second receptacle and the outer receptacle must have a specimen list. If the presence of category A infectious substances is suspected, “(suspected category A infectious substances)” shall be shown.
4. Complete the testing form in Annex 6 enclosed with this Circular and enclosed it with the specimen.
Article 8. Transport of specimens
1. The unit in charge of specimen collection shall notify the laboratory of the shipping date of the specimen, the means of transport, and the expected arrival time at the laboratory.
2. The unit in charge of specimen collection shall select the means of transport and ensure the shortest transport period. This period must not exceed the storage period prescribed in Article 5 of this Circular.
3. Means of transport: by air, by sea, by road, by rail
a) Air transport:
- For specimens containing category A infectious substances:
+ Up to 50 ml or 50 g per package for passenger aircraft;
+ Up to 4 litre or 4 kg per package for cargo aircraft.
- For specimens containing category B infectious substances:
+ For liquid: the volume of a specimen shall not exceed 1 litre, the volume of a specimen package shall not exceed 4 litres;
+ For solids (except for body parts and organs): the weight of a package of specimen shall not exceed 4 kg.
b) Road, rail, and sea transport: no limit on the weight of each package of specimen.
1. The affected are shall be washed or disinfected as soon as possible, regardless of the agent.
2. When a infectious substance comes into contact with non-intact skin, it is recommended to wash with soap and water or with and antiseptic solution, and go to a nearest medical facility for advices and treatment.
3. Spill clean-up procedure:
a) Block the spillage area;
b) Wear gloves, protective clothing, mask, or glasses depending on the infectious substances;
c) Cover the spill with a cloth or blotting paper;
d) Pour an appropriate disinfectant over the cloth or blotting paper (beginning at the outer margin towards the center);
dd) After about 30 minutes, dispose of contaminated materials into a leak-proof waste disposal container (use a puncture-resistant container if broken glass or other sharps are involved)
4. After the disinfection, report the incident to the leader of the carrier.
Article 10. Receipt, testing, and storage of specimens
1. After being informed by the consignor, the laboratory that receives the specimens shall arrange for the timely receipt. The date, time of receipt, the consignee, and the status of the specimen received must be recorded upon the receipt.
2. Conduct the testing in accordance with the technical procedure for each type of specimen and testing purpose.
3. The specimens, specimens of virus and bacteria serving research must be stored in accordance with the regulations on each infectious substance. The temperature and status of specimens must be regularly checked.
Article 11. Destruction of specimens
The specimens that are used, studied, or expired must be destroyed in accordance with the Decision No. 43/2007/QD-BYT dated November 30th 2007 on the Regulation on medical waste, and relevant laws.
DOCUMENTATION AND PROCEDURE FOR THE IMPORT AND EXPORT OF SPECIMENS
Article 12. Dossier of application for the import or export of specimens
1. The application form for the import or export of specimens according to the template in Annex 7 enclosed with this Circular.
2. The documentations proving the necessity of the import or export of specimens, including the photocopy of the written approval issued by a ministerial or provincial competent authority for the research or project, and the outline of the research or project that is approved of, or the written agreement on the cooperation between a Vietnamese party and a foreign party relating to the import or export of specimens.
3. The photocopy of the Certificate of Business registration, Establishment Decision or other papers proving that the organization is licensed to export, import, research, preserve, transport, and test specimens.
4. Where an organization licensed to research as prescribed in Clause 1 Article 3 of this Circular authorizes another organization licensed to import or export as prescribed in Clause 2 Article 3 of this Circular to import or export specimens, the dossier must contain the contract signed by both of them.
Article 13. Procedure for submitting and settling applications for the import or export of specimens
1. The applicant for the import or export of specimens shall send a dossier to the Ministry of Health (the Department of Defensive Medicine)
2. Within 10 working days from the day on which the complete and valid dossier is received, the Department of Defensive Medicine shall issues a written decision to grant or not to grant the License to import or export specimens.
CONDITIONS FOR THE MANAGEMENT OF SPECIMENS CONTAINING CATEGORY A INFECTIOUS SUBSTANCES
1. The organizations that management specimens containing category A infectious substances must have the functions prescribed in Clause 1 Article 3 of this Circular, and meet the standards of Laboratory Biosafety Level III according to the Government's Decree No. 92/2010/ND-CP dated August 30th 2010 on the implementation of the Law on Prevention and suppression of the Ministry of Natural Resources and Environment, applicable to laboratory biosafety, and relevant guiding documents.
2. The laboratories that meet the standards of biosafety level II may only temporarily store specimens containing category A infectious substances, then send them to a laboratory that meet the standards of biosafety level III as soon as possible.
Article 15. The implementation
This Circular takes effect on January 15th 2012.
Article 16. Responsibility to organize the implementation
1. The Department of Defensive Medicine shall receive applications and cooperate with other agencies in considering granting Licenses to export and import specimens related to infectious substances; periodically and randomly inspect the research, store, transport, retain, and destruction of specimens.
2. The Department of Medical Examination and Treatment and the Science and Education Department shall cooperate with the Department of Defensive Medicine in providing instructions and inspecting the research, use, and storage of specimens.
3. The Inspectorate of the Ministry of Health shall cooperate with competent agencies affiliated to the Ministry of Health in carrying out inspections of the import, export, transport, preservation, storage, use, research, exchange, and destruction of specimens related to infectious substances nationwide.
4. The Inspectorate of the Ministry of Health shall cooperate with competent agencies affiliated to the Ministry of Health in carrying out inspections of the import, export, transport, storage, retention, use, research, exchange, and destruction of specimens related to infectious substances nationwide.
5. The epidemiology institutes, provincial defensive medicine centers, hospitals, organizations and individuals licensed to store, retain, use, research, import, export, transport, and destroy specimens related to infectious substances shall comply with law provisions and be responsible for their acts.
|
| THE MINISTER |
LIST OF CATEGORY A INFECTIOUS SUBSTANCES
| No. | UN number | MICROORGANISM |
|
| UN 2814 Infectious substances affecting humans | Bacillus anthracis (cultures only) Brucella abortus (cultures only) Brucella melitensis (cultures only) Brucella suis (cultures only) Burkholderia mallei – Pseudomonas mallei (cultures only) Burkholderia pseudomallei – Pseudomonas pseudomallei (cultures only) Chlamydia psittaci – avian strains (cultures only) Clostridium botulinum (cultures only) Coccidioides immitis (cultures only) Coxiella burnetii (cultures only) Crimean-Congo haemorrhagic fever virus Dengue virus (cultures only) Eastern equine encephalitis virus (cultures only) Escherichia coli, verotoxigenic (cultures only)1 Ebola virus Flexal virus Francisella tularensis (cultures only) Guanarito virus Hantaan virus Hantaviruses causing haemorrhagic fever with renal syndrome Hendra virus Hepatitis B virus (cultures only) Herpes B virus (cultures only) Human immunodeficiency virus (cultures only) Highly pathogenic avian influenza virus (cultures only) Japanese Encephalitis virus (cultures only) Junin virus Kyasanur Forest disease virus Lassa virus Machupo virus Marburg virus Monkeypox virus Mycobacterium tuberculosis (cultures only)1 Nipah virus Omsk haemorrhagic fever virus Poliovirus (cultures only) Rabies virus (cultures only) Rickettsia prowazekii (cultures only) Rickettsia rickettsii (cultures only) Rift Valley fever virus (cultures only) Russian spring-summer encephalitis virus (cultures only) Sabia virus Shigella dysenteriae type 1 (cultures only)1 Tick-borne encephalitis virus (cultures only) Variola virus Venezuelan equine encephalitis virus (cultures only) West Nile virus (cultures only) Yellow fever virus (cultures only) Yersinia pestis (cultures only) Virus causing severe acute respiratory syndrome
|
1 For surface transport (ADR), when the cultures are intended for diagnostic or clinical purposes, they may be classified as infectious substances in category B.
The list of category A infectious substances shall by updated at the request of the Department of Defensive Medicine.
SYNDROMES AND SPECIMENS TO BE COLLECTED
| No. | Syndrome | Specimens required (*) |
| 1 | gastrointestinal infection | stool and vomit |
| 2 | Respiratory tract infection | throat swab, nasopharyngeal swab, sputum, serum |
| 3 | CNS infection | stool, throat swab, serum, cerebrospinal fluid |
| 4 | Bloodstream infection | serum and blood |
| 5 | Urinary tract infection | urine |
| 6 | Skin and mucous membrane infection | vesicular fluid, blood, serum, throat swab |
| 7 | Jaundice | serum and blood |
(*) Doctors may indicate the type of specimen to be taken and request the testing on that specimen on a case by case basis.
1. Collection of nasopharyngeal swab:
1.1. Instruct the patient to tilt the face backwards to about 45 degrees.
1.2. Insert the swab along the floor of nose into the nasopharyngeal space. Let the swab stick absorb the nasopharyngeal fluid, then rotate and rub it against the nose and slowly remove the swab.
1.3. After nasopharyngeal fluid is collected, the swab stick shall be stored in a protective medium for specimens (the swab head must be submerged in the transport medium). The swab stick handle shall be cut down to fit the length of the viral transport medium container.
1.4. Firmly tighten the cap and cover the container with parafilm (if available).
2. Collection of throat swab:
2.1. Instruct the patient to open his/her mouth widely.
2.2. Use a tongue depressor to depress the patient’s tongue.
2.3. Put a swab stick into the pharynx region and let the throat swab. Then rub and rotate the swab stick in the tonsillar region and the throat to collect infected cells.
2.4. After that, the swab stick shall be stored in a protective medium for specimens (the swab head must be submerged in the transport medium). The swab handle shall be cut down to fit the length of the transport medium container.
2.5. Firmly tighten the cap and cover the container with parafilm (if available).
3. Serum specimen collection:
3.1. Use a sterile syringe to take 3 – 5 ml of venous blood, then remove the needle, lean syringe against the test tube and slowly transfer blood to the tube to avoid air bubbles and haemolytic. Tighten the cap and store at 4 oC within 24 hours.
3.2. Serum shall be separated as follows:
- Tighten the cap of the blood tube and centrifuge it at 2000 round per minute for 8 minutes to keep erythrocytes from being broken. Do not freeze the blood specimen before serum is separated.
- Use a sterile pipette to gently take serum from the upper part of the tube and evenly distribute to smaller tubes (1.8 ml) for storage.
3.3. Serum shall be stored at 4 – 8 oC within 19 days. For longer periods, they must be stored or at -70oC or -20oC.
4. Stool specimen collection:
4.1. Collect fresh stool, 5 ml liquid or 4 – 5 g solid (pea-size) in a container.
4.2. Method of collecting a rectal swab from infants:
- Moisten a swab stick in sterile saline
- Insert the swab tip just past the anal sphincter and rotate gently
- Remove the swab stick and examine to ensure that the swab tip is stained with stool
- Place the swab in the tube that contain appropriate bacterial or viral transport medium
- Break off the top part of the swab stick without touching the tube, and then firmly tighten the screw cap
5. Urine specimen collection:
5.1. Materials for collection:
- Sterile plastic cup (≥ 50 ml)
- Screw-cap containers
- Gauze pads
- Soap and clean water
5.2. Method of collection
- Give the patient clear instructions to use the cup to take urine after a few second to catch a mid-stream urine sample in order to decrease the risk of contamination from organisms living in the urethra. To decrease the risk of contamination from skin organisms, the patient should pass urine directly into the plastic cup, avoid touching the inside or rim of the plastic cup with the hands or legs.
- For hospitalized or debilitated patients, it may be necessary to wash the external genitalia with soap and clean water to reduce the risk of contamination. If soap and clean water are not available, the area may be rinsed with saline. Use gauze pads to dry the area before collecting the urine.
- Urine collection bags may be necessary for infants. If used, urine must be transferred from the urine bag to urine containers as soon as possible to prevent contamination with bacteria. A sterile pipette may be used to transfer the urine to the container.
- Label the urine container. Ensure that the urine container is tightly sealed and leak-proof.
6. Cerebrospinal fluid collection
6.1. Materials for collection:
- Tray
- Sterile materials: gloves, gauze pads
- Local anaesthetic, needle, syringe
- Skin disinfectant: 10% povidone iodine or 70% alcohol
- Puncture needles with stylet
- 06 screw-cap tubes and tube rack
- Water manometer
6.2. Method of collection
Only experienced persons are allowed to collect cerebrospinal fluid. Cerebrospinal fluid shall be put into separate tubes.
7. Skin specimen collection:
For most dermatological diseases, symptoms are determined on the basis of physical examination and clinical history without the collection of diagnosis specimens. Important characteristics to be noted on physical examination include the nature of the lesions (erythema, maculae, papulae, vesicles, etc.) and the distribution of skin lesions. In cases of indeterminate diagnoses, unusual presentations and some rare conditions, collection of specimens from rashes and/or skin lesions may be necessary. In the case of vesicles, specimens for microscopy and culture are taken directly from vesicles. In other exanthemata and papulae, the diagnosis may be established from other specimens (blood cultures, serology). In suspected anthrax or bubonic plague, specimens from the skin lesions and blood cultures shall be taken.
Materials for skin specimen collection:
- Sterile saline
- Sterile swabs and appropriate transport media
- Sterile screw-cap vials
- Sterile lancets or needles
- Wide-bore needle and syringe
- Wide-mouth screw-cap containers
- Glass slides and slide boxes
1. Specimens for viral isolation may be stored in appropriate transport media at 4 – 8oC up to 48 hours. For longer periods, these specimens shall be stored at -700C, or -200C if a -700C freezer is not available. Avoid unnecessary freeze-thaw cycles to prevent the microorganisms therein from losing their activity. Serum may be stored at 4oC - 8oC up to 10 days, or at -20oC for longer periods.
2. Specimens for isolating influenza virus shall not be stored or transported in solid carbon dioxide, unless they are sealed in glass tubes or in 2 layers of plastic bags.
3. Specimens for bacterial culture should be kept in appropriate environment and at suitable temperature in order to ensure the bacterial viability while minimizing the growth of other microorganisms. Except for sputum and urine, other specimens may be kept at ambient temperature within 24 hours. For longer periods, they must be stored at 4-8oC, except for cold-sensitive microorganisms such as shigella, meningococcus and pneumococcus. Avoid prolonged storage as the yield of bacteria may fall significantly.
4. Specimens for antigen or antibody detection may be stored at 4-8oC within 48 hours, or at -20oC for longer periods. Some specimens may require special handling, for example freezing. Therefore instructions on the storage should be provided prior to the specimen collection. Serum specimen for antibody detection may be stored at 4-8oC for up to 10 days. Avoid unnecessary freeze-thaw cycles. Adequate information about the specimen storage and transport conditions.
5. Stool specimen shall be stored at 4-8oC. The quantity of bacterial may fall significantly if specimens are not properly processed within 1 0 2 days of collection. Shigella are particularly sensitive to high temperature.
Stool specimens used for detection of parasites should be mixed with 10% formalin or PVA, 3 parts stool to 1 part preservative. Specimens containers shall be sealed in plastic bags and transported at ambient temperature.
6. Urine specimens must be transported to the laboratory within 2 – 3 hours. If this is not possible, store the specimens at 4-8oC and avoid freezing them.
LABELS FOR THE TRANSPORT OF INFECTIOUS SUBSTANCES
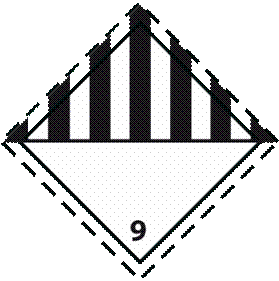
1. Label 1: Used for specimens containing category A infectious substances.
Label name: infectious substance
Minimum dimensions: 100 x 100 mm (for small packages: 50 x 50 mm)
Number of labels per package: 1
Color: Black and white

2. Label 2: Used for specimens containing category B infectious substances
Label name: Category B infectious substance
Minimum dimensions (for air transport): 50 x 50 mm
Minimum height of letters and numbers: 6 mm
Color: none specified, contrasting the color of the outer packaging
The words "BIOLOGICAL SUBSTANCE, CATEGORY B" shall be at least 6 mm high

3. Label 3: Used for noninfectious generically modified organisms, carbon dioxide, dry ice, and substances packed in dry ice. Label 3 is used in addition to Label 1 or Label 2
Label name: Miscellaneous dangerous substances
Minimum dimensions: 100 x 100 mm (for small packages: 50 x 50 mm)
Number of labels per package: 1
Color: Black and white
4. Label 4: Used for liquid nitrogen and substances packed using liquid nitrogen Label 4 is used in addition to Label 1 or Label 2
Label name: non-flammable, non-toxic gas
Minimum dimensions: 100 x 100 mm (for small packages: 50 x 50 mm)
Number of labels per package: 1
Color: Green and white or green and black

5. Label 5: Used for cryogenic liquids, for transport by air, for deeply refrigerated liquefied gases. Label 5 is used in addition to Label 1, 2, or 4 as appropriate.
Label name: Cryogenic liquid
Minimum dimension: 74 × 105 mm
Number of labels per package: 1
Color: Green and white

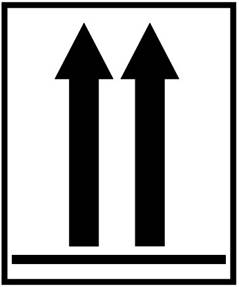
6. Label 6: Used to indicate the position of closures on the primary receptacles. It is used when the volume of specimens containing category A infectious substances in each primary receptacle exceeds 50 ml while being transported by air.
Label name: Orientation label
Minimum dimension: 74 × 105 mm
Number of labels per package: 2 (on opposite sides)
Color: Black and white or red and white
---------------------------------------------------------------------------------------------------------------------------------
- 1Decree No. 92/2010/ND-CP of August 30, 2010, detailing the implementation of the Law on infectious disease prevention and control, regarding biosafety in laboratories
- 2Decree No. 22/2010/ND-CP of March 09, 2010, amending and supplementing Article 3 of the Government''s Decree No. 188/2007/ND-CP of December 27,2007, defining the functions, tasks, powers and organizational structure of the Ministry of Health
- 3Decree No. 188/2007/ND-CP of December 27, 2007, defining the functions, tasks, powers and organizational structure of the Ministry of Health.
- 4Decision No. 43/2007/QD-BYT of November 30, 2007, promulgating the regulation on management of medical wastes.
- 5Law No. 03/2007/QH12 of November 21, 2007, on prevention and control of infectious diseases.
Circular No. 43/2011/TT-BYT of December 05, 2011, on the management of infectious specimens
- Số hiệu: 43/2011/TT-BYT
- Loại văn bản: Thông tư
- Ngày ban hành: 05/12/2011
- Nơi ban hành: Bộ Y tế
- Người ký: Nguyễn Thị Kim Tiến
- Ngày công báo: Đang cập nhật
- Số công báo: Dữ liệu đang cập nhật
- Ngày hiệu lực: 20/01/2012
- Ngày hết hiệu lực: 12/11/2018
- Tình trạng hiệu lực: Kiểm tra

